
The application is open to any new Indian entity that wishes to manufacture biological goods in India.
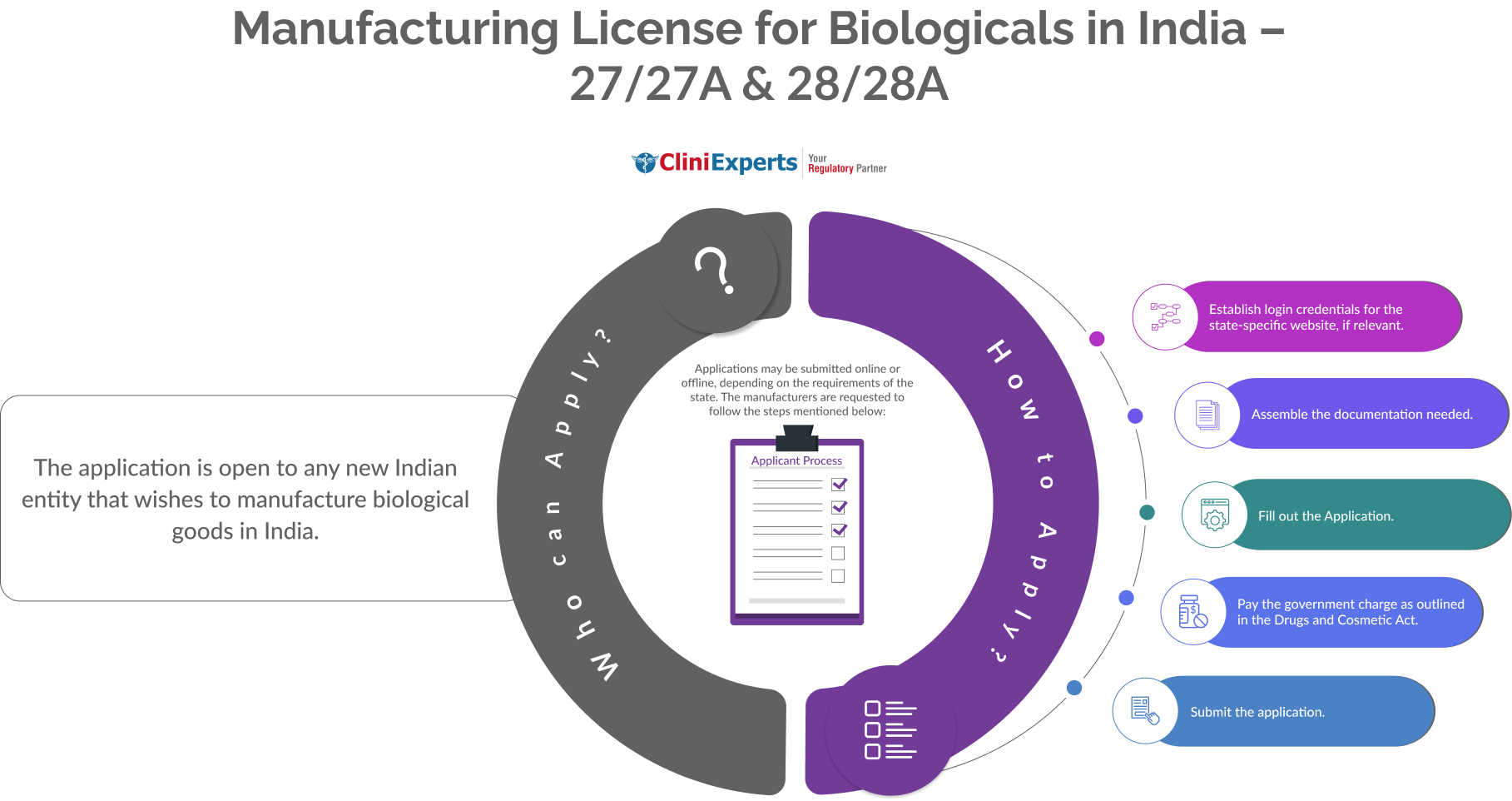
The Applicant must follow the following process:
 |
Establish login credentials for the state-specific website, if relevant. |
 |
Assemble the documentation needed. |
 |
Fill out the application. |
 |
Pay the government charge as outlined in the Drugs and Cosmetic Act. |
 |
Submit the application. |

The license validity for manufacturing biological products is 5 years.

There is a government fee of Rs. 7500 for the application process.
28/28Afrom Central Drugs Standard Control Organisation
60
DaysFacility should comply the Schedule M1 requirement.
NOC from the pollution Control Board & fire department is must.

During the documentation process, the following documents should be present:

There is a government fee of Rs. 7500 for the application process.


No, the manufacturers must obtain product permissions and may also seek GMP certification.
