
The application is open to any manufacturer of Medical Devices in India.
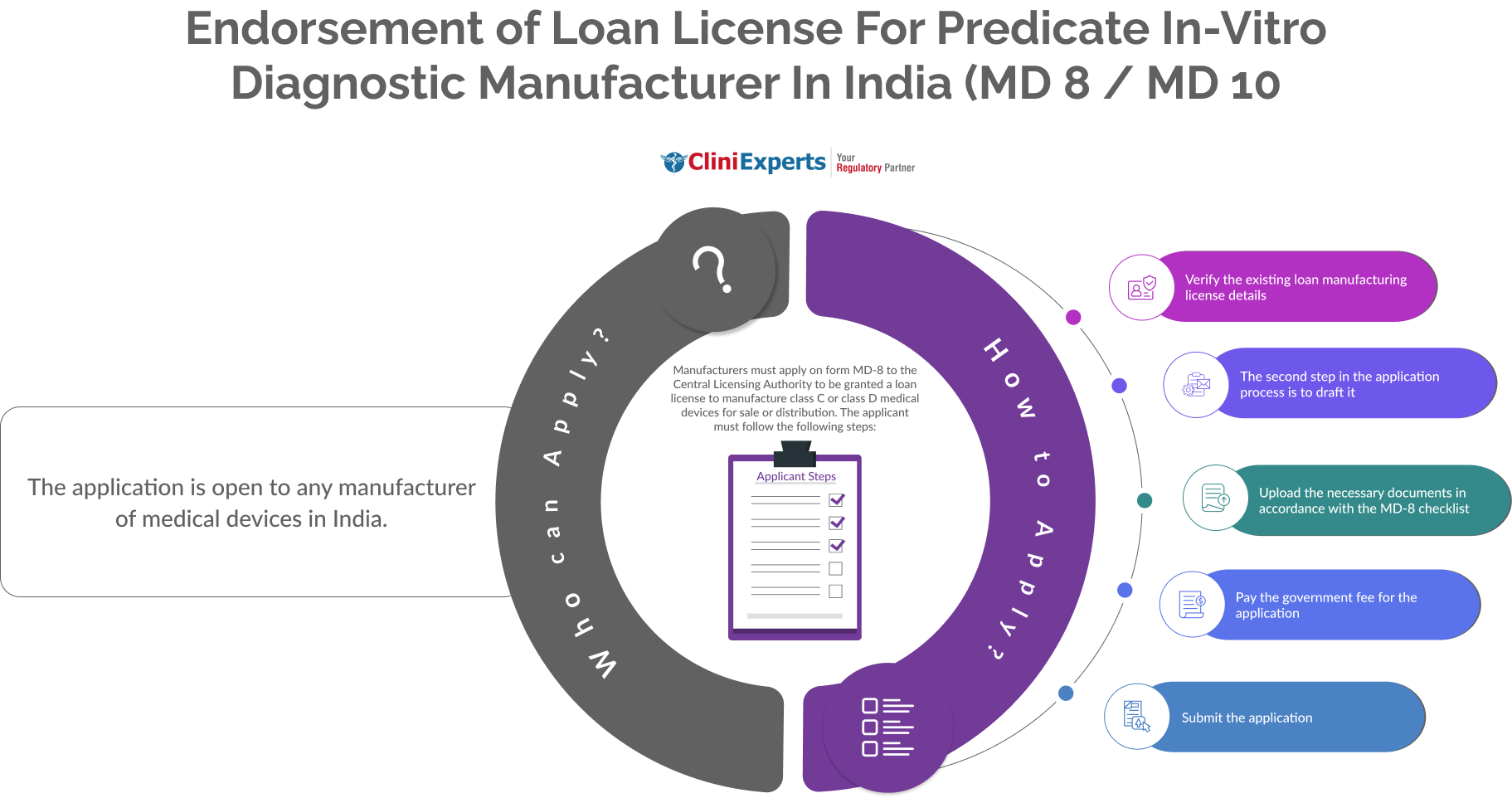
The Applicant must follow the following process:
 |
Verify the existing loan manufacturing license details. |
 |
The second step in the application process is to draft it. |
 |
Verify the existing loan manufacturing license details. |
 |
Upload the necessary documents in accordance with the MD-8 checklist. |
 |
Pay the government fee for the application. |
 |
Submit the application. |


MD 10from Central Drugs Standard Control Organisation
4-6
MonthsAccording to our experts, the manufacturers must generate the quality control data using a valid test license at the time of application submission.
The applicants can expect the inspection by CDSCO within sixty days from the date of submission of the application.


If an IVD manufacturing facility already has a license to produce the Medical Device for sale or distribution, no inspection of the facility is necessary to award a loan license to manufacture the device.
